Current Situation of Emerging Technologies for Upgrading Heavy Oils
Author: Marcello De Falco, Associate Professor, University UCBM – Rome (Italy)
1.Theme description
The change in the average crude oil quality due to the scarcity of light oil reserves and to the increase of the use of shale oil, oil sand and bitumen is causing significant troubles to refineries, which are obliged to accept heavier feeds with very different physical properties (lower API gravity, higher amount of impurities). This has stimulated the development of new technology for upgrading the heavy and extra-heavy oils in order to improve their characteristics and, consequently, the refineries performance.
Heavy oils are classified as oil with a API gravity within the range 10°- 22°, whereas extra-heavy oils have a API gravity < 10°. Heavy oils and bitumen reserves geographical distribution is reported in Table 1 [1]: these reserves are continuously increasing, replacing the light oil ones, and Oil&Gas companies found and developed competitive solutions to extract and treat these oils.
Table 1 – Geographical distribution of heavy oil and bitumen reserves.
Clearly, there is the need to upgrade the heavy oils before feeding to the refineries, in order to improve the downstream products quality and to increase the topping distillate flows: the conventional upgrading processes include carbon rejection and hydrogen addition technologies. But, when the properties of the heavy and extra-heavy oils are critical, more effective solutions are needed to make the oil suitable for refineries feedstock. For this reason, researchers and industries are proposing a number of innovative solutions and some of them are already in the full-scale demonstration phase.
In the following, some of these new emerging oil upgrading configuration are presented. For a complete list of developed technologies, the author suggests the Castaneda, Munoz and Ancheyta review paper[2], which includes the description and comparison of 23 new processes.
2.Heavy-to-Light heavy oil upgrading process
The Heavy-to-Light (HTL) process is patented by Ivanhoe Energy[3], a company recently acquired by FluidOil[4].
The process is based on a circulating transport bed of hot sand to heat the heavy feedstock and convert them to lighter products. Then the upgraded products and the sand are separated in a cyclone and the products are quenched and routed to the atmospheric distillation unit.
The main benefits of the HTL configuration are that it can be integrated at the well-head and it is simple and cheap. The drawbacks are the large dimension of the equipment, the low volumetric yield of upgraded crude, the low capacity for extra-heavy oil processing, the high formation of coke and a low sulfur content reduction. At the exit of the upgrading plant, the oil reaches a API gravity of 18-19° and a 100°C kinematic viscosity of 23 cSt.
The technology development has been completed and Ivanhoe Energy is designing industrial plants in Canada, Latin America and the Middle East.
Figure 1 – Ivanhoe Energy’s HTL test facility in San Antonio, Texas[5].
3.HCAT process
HCAT is a catalytic heavy oil upgrading technology developed by Headwaters Technology Innovations Group (HTIG)[6]. The process is composed by a catalytic reactor where a molecule sized catalyst is packed, assuring high conversion of the heavy oil. The main benefits of HCAT configuration are constant product quality, feedstock flexibility and flexible and high conversion (up to 95%).
Neste Oil Corporation’s Porvoo Refinery at South Jordan, Utah, is the first refinery which implements, in 2011, the HCAT heavy oil upgrading technology[7]. More than 500.000 barrels of heavy oil are processed in their upgrading reactors every day and a refinery additional capacity of 200.000 barrels per day has been reached.
4.Viscositor process
Viscositor technology is patented by the Norwegian company Ellycrack AS[8] and it is based on the atomization of the heavy oil by means of a heated sand in a high-velocity chamber. Basically, the process is composed by the following steps (refer to the block diagram shown in Figure 2):
- sand particles, heated up by coke combustion, are pneumatically conveyed into a collision reactor by means of the hot combustion gases;
- pre-heated heavy oil is fed to the reactor and collide with the sand particles, evaporating and cracking;
- the solid particles, the coke generated during the collision process and the oil stream are separated in a cyclone. The solid phase is sent to a regenerator while the oil stream is fed to a dual condensation system. The generated coke is used to supply the heat duty.
The advantages of the process are the low temperature and the low pressure required, almost self-sustained thanks to the coke formation in the reactor and the good quality of the final upgraded oil.
Figure 2 – Block diagram of the Viscositor process
5.IMP process
IMP configuration[9] is a catalytic hydrotreatment-hydrocracking process of heavy oil at mild operating conditions, able to achieve high removal of metals, sulfur compounds, asphaltenes and a large conversion of the heavier share of the oil stream to more valuable distillates.
The most important characteristic of the IMP process is the low fixed investment required and the low operating costs, with an attractive return of investment.
The IMP technology can be applied both for conversion of heavy and extra-heavy oils to intermediate oils and as a first processing unit for heavy and extra-heavy crude oils in a refinery. The final properties of upgraded oils, depending on the heavy oil feedstock, are: API gravity = 22-25°; sulfur content = 1.1 – 1.15 wt%; C7 asphaltenes = 4.7 – 5.3.
A first industrial unit application is being analyzed by Petroleos Mexicanos (PEMEX).
6.Nex-Gen Ultrasound technology
Nex-Gen is an innovative process for heavy oil upgrading which uses ultrasonic waves to break the long hydrocarbon chains and simultaneously adds a hydrogen stream.
Basically, Nex-Gen is a cavitation process: the ultrasonic energy forms cavitation bubbles in the heavy oil stream; then, the bubbles tends to collapse at high temperature and pressure, causing the breaking of the long chain of heavy hydrocarbon molecules.
The next figure shows a scheme of the Nex-Gen configuration.
Figure 3 – Scheme of NexGen architecture[10].
A first industrial plant is going to be designed to be integrated near the Athabasca tar sands (Edmonton, Alberta), with a capacity of 10.000 barrels per day. The mild operating conditions (temperature = 0 – 70°C, pressure = 1 – 5 bar) allows a reduction of energy consumption and operating and maintenance costs by 50%.
7.Chattanooga process
Chattanooga process is a continuous process based on a fluidized bed reactor operating at high pressure and temperature in a hydrogen environment.
The main equipment of the configuration is the pressurized fluid bed reactor and associated fired hydrogen heater. The reactor can continuously convert oil by thermal cracking and hydrogenation into hydrocarbon vapors while removing spent solids[11].
The energy requirements associated with the Chattanooga configuration are significantly reduced in respect to the traditional heavy oil upgrading technologies, as well as operating costs and capital costs.
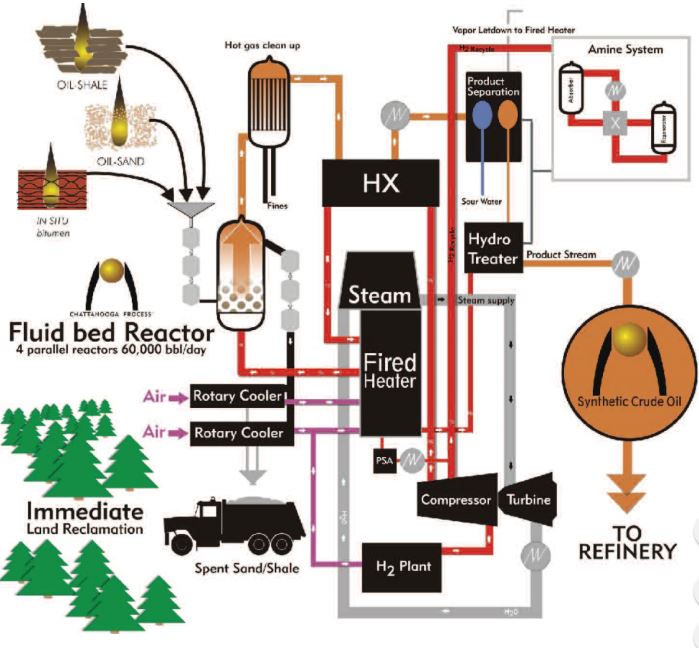
Figure 4 – Chattanooga technology description11.
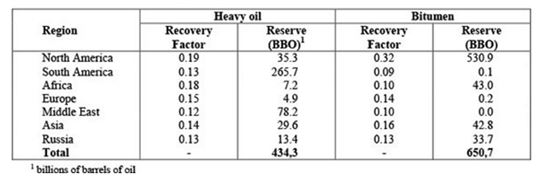

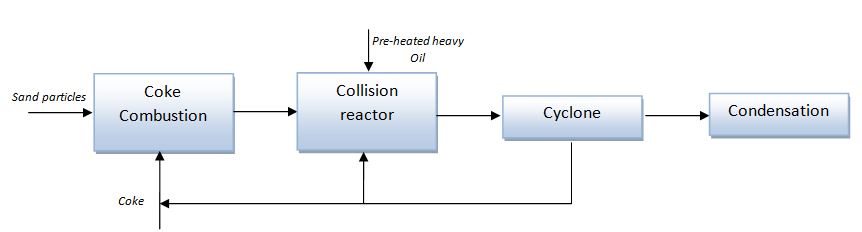
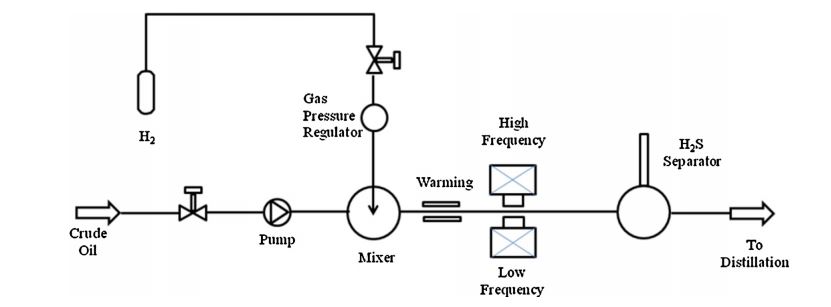











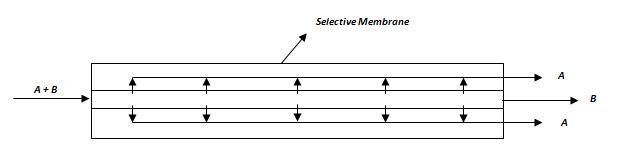
 Figure 2 – Composite membrane architecture
Figure 2 – Composite membrane architecture



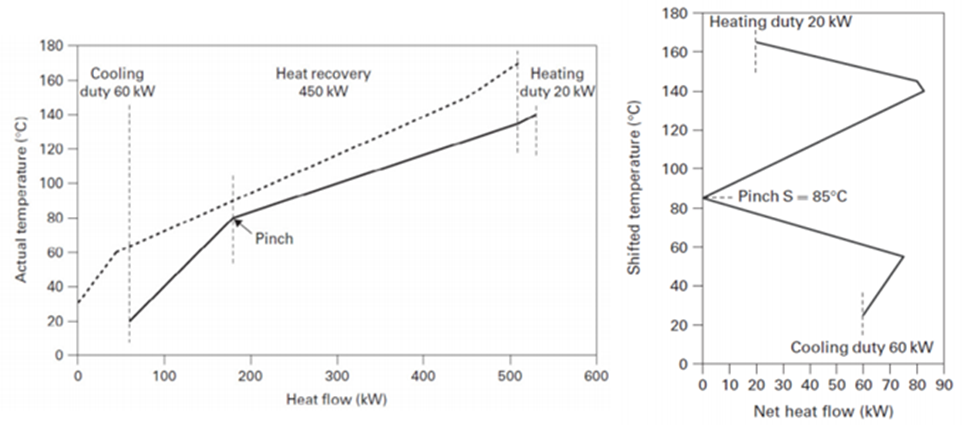
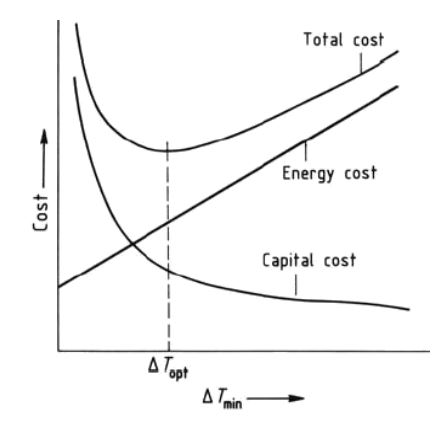 improvement, but has the aim to match the cold and hot process streams with a HEN that minimize the external energy supply. According to the PA fundamentals, the first step is to draw the heating and cooling curves to evaluate the minimum temperature difference ΔTmin and the related energy target corresponding to reasonable values of the temperature differences.
improvement, but has the aim to match the cold and hot process streams with a HEN that minimize the external energy supply. According to the PA fundamentals, the first step is to draw the heating and cooling curves to evaluate the minimum temperature difference ΔTmin and the related energy target corresponding to reasonable values of the temperature differences.