Research Highlights in New Catalytic Technologies
Author: Mauro Capocelli – Researcher –University UCBM – Rome (Italy)
1.Theme Description
Catalysts are compounds use for increasing the velocity of a specific reaction by reducing the activation energy.[1] This brings down temperature/pressure of the processes saving fuel. Catalysts can be homogenous or heterogenous depending on the phase involved in the reactions (i.e. heterogenous catalysts usually are solid while the reagents are liquid or gaseous).[2] These substances are not reduced by the reactions, but over time catalytic activity and selectivity decrease due to phenomena such as poisoning, fouling coking, carbon deposition and sintering.[3] Therefore regeneration is necessary. In 2014 the global market for catalysts and catalysts regeneration reached 24.6 Billion of US$[4]and it is estimated to achieve 34.3 Billion of US$ in 2024.[5]

Figure 1– Common catalysts use in refining and petrochemical processes[6]
Nowadays the main companies are: Haldor Topsoe, UOP, Johnson Matthey, Süd-Chemie, BASF, Exxon Mobil Chemical and so on.[7] In the following sections catalyst technologies in chemical and refining sectors are described. In addition, new trends in the development of catalysts are illustrated.
2.Catalysts in Industrial Processes
Catalysts are widespread into Industrial Processes, from chemical to refining sectors, for the production of several compounds.
2.1 Chemical Sectors
In the chemical sectors catalysts are used to get:
- Xylenes a mixture of aromatic hydrocarbon molecules obtained from petroleum naphtha and to lesser extent from pyrolysis gasoline (by-product from ethylene plant) and coal liquids from coke.[8] The mixture is rich in m-xylene (50-60%), but the most important is para-xylene (20-25%)[9] used for the production of polyester fibres[10], resin and films. Referring to p-xylene is usually obtained by crystallization or adsorption on molecular sieve processes,[11] but it’s difficult to separate it from other isomers (very close boiling points). Therefore, toluene disproportionation and methylation are also used. Both technologies exploit zeolite catalysts (i.e. ZSM-5) and allow to have high selectivity to p-xylene. Toluene disproportion produces, from two molecules of toluene, one of xylene and one of benzene while the toluene methylation produces, from the reaction of toluene and methanol, water and xylenes.[12]
- Ethylbenzene is used for the production of styrene, a chemical compound employed to synthetize thermoplastic polymers and elastomers (8) Usually, ethylbenzene is obtained by means of alkylation where ethylene reacts with benzene on acid catalysts. Transalkylation is also used to improve process yield by converting polyalkylbenzenes (PBE), a by-product, into ethylbenzene.[13]Figure 2illustrates the process developed by Polimeri Europa (now Versalis). The system uses the proprietary zeolite Beta-based catalysts: PBE-1 for the alkylation section and PBE-2 for the transalkylation section.[14]
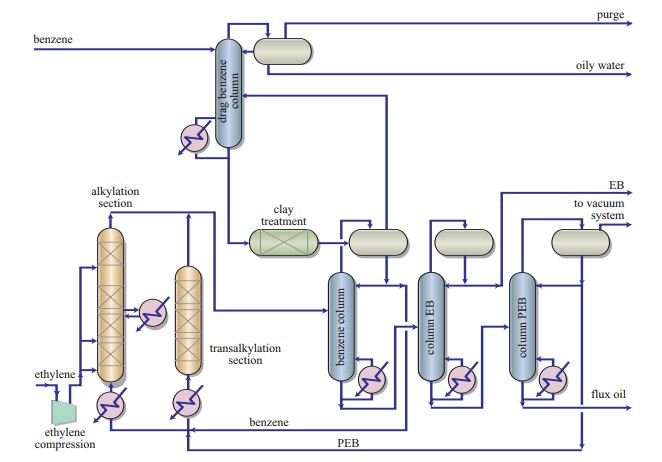
Figure 2 – Production of Ethylbenzene from Polimeri Europa (Versalis since 2012)(8)
- Cumene is used for phenol and acetone production. It is obtained from the catalytic alkylation of benzene with propylene. The most common catalysts are based on Solid PhosphoricAcid (SPA)housed in fixed-bed reactors operating at 180-240°C and 3-4 MPa. However, the release of free acids causes problems of corrosion.8Hence, new zeolite-based catalysts have recently been launched. The UOP, for example, has developed the Q-MaxTM Process[15] shown in Figure 2, where zeolite catalysts are used for both alkylation and transalkylation reactors. These compounds are regenerated after three cycles.

Figure 3 – Q-MaxTM Process developed by UOP15
In the process, a depropanizer and diisopropylbenzene (DIPB) column are used. The former allows to remove propane from alkylation reactor effluent while the last separates DIPB from heavy aromatics. A transalkylation reactor, in which DIPB reacts with benzene, is also used to improve the yield of cumene.
2.2 Refining
Catalyst technologies are used in refining processes such as:
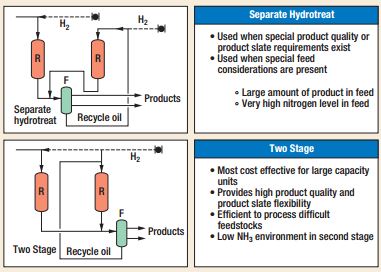
- Hydrotreating used to remove sulphur, nitrogen, oxygen olefin and metals from distillate fuel such as naphtha, diesel, kerosene by means of hydrogen at high pressure and temperature by means of catalysts. These cylindrical catalysts are metal oxide-based (NiMo,CoMo or MoO3, WO3) on alumina supports.[16] Table 1 summarizes the main physical properties.
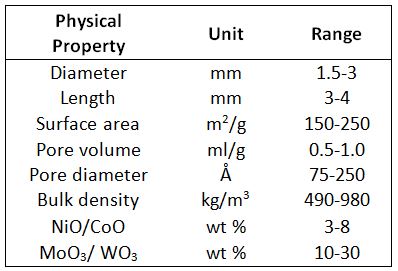
Table 1– Physical Property of NiO/CoO and MoO3/WO3 catalysts (values from[17])
- Catalytic Reforming is used to transform heavy naphtha in gasolinewith high octane ratings by means of fixed bed reactors. Catalysts can be platinum-based or mixtures of it on alumina support (Pt/Al2O3, Pt-Re/Al2O3, Pt-Ti/Al2O3).[18] Before catalytic reforming the feed is hydrotreated to remove sulphur and nitrogen that poison the active catalyst sites.
- Isomerization of light naphtha allows to improve octane ratings of C5,C6 hydrocarbon up to 10-20 times. Chlorided alumina, zeolite, and sulfated oxide are the most common catalysts. The first one has high activity and high isomerate yields, but is sensible to poisoning, hence chloride addition is necessary. Zeolite and sulfated oxide can be regenerated but has less activity and require high H2/hydrocarbons ratios.[19]
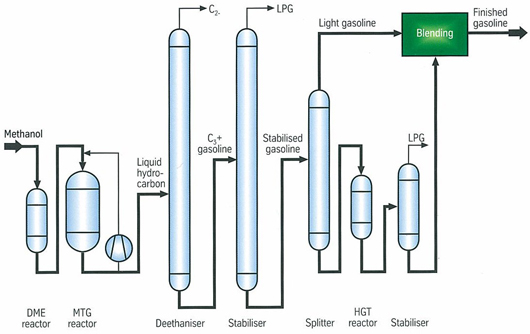
- Synthetic Fuels are obtained from syngas, a mixture of CO+H2. In Fischer-Tropsch Synthesis, syngas is converted into hydrocarbons blends that are further refined to produce gasoline. The process uses transition metal catalysts such as iron or cobalt. In the presence of iron catalysts, the water produced from the reactions are converted in CO2 e H2 by means of water gas shift reaction. The temperature and operating pressure are between 200-350°C and 20-50 bar respectively. Syngas is also used to produce methanol by means of catalysts such as Cu/ZnO/Al2O3 at 225-275°C and 50-100 bar[20].ExxonMobilhas developed a process to convert methanol to gasoline (MTG), as shown in the Figure 4, methanol is vaporized and introduced into a DME reactor. The effluent, a mixture of methanol/DME, is sent to MTG reactors in which is completely dehydrated by means of own catalysts, producing gasoline. Gasoline enters Deethanizer and Stabilizer reactors where fuel gas and LPG fractions are removed. Then, stabilized gasoline is split into light and heavy gasoline; the last stream is treated to reduce the amount of Durene.
Figure 4– Methanol to Gasoline ExxonMobil Process[21]
- Catalytic Dewaxing is a process that improves cold flow of middle distillate feedstocks. Commercially there are two different configurations depending on the catalysts used. For example, if catalysts are based on Ni, Co/Mo or Ni/Mo, a single stage is adopted. The stacked beds of hydrotreating(to remove sulphur and nitrogen) and dewaxing are placed on the top/bottom of the reactor according to the raw materials. Whereas, in the presence of noble-metal catalysts, a double stage is used because a severe hydrotreating is required. The former consists of stacked beds of hydrotreating while the last is a dewaxing stage[22]. Shell has developed a proprietary catalyst formulation (SDD),for dewaxing stage, that allows to remove “wax” converting it in isomerized and cracked molecules. The single stage uses SDD-800 that reduces the loss of distillate and increases the activity of the catalyst before regeneration. The catalyst can operate under high concentration of H2S and NH3. The double stage, instead, adopts SDD-821 a noble metalcatalyst that increases yield, but require slow percentages of H2S and NH3.[23]
3.R&D in Catalytic Technologies
Catalysts allow to reduce the temperature/pressure of the reaction decreasing the amount of fuel, feedstock and expensive materials involved in the processes. Therefore, is crucial to develop new catalysts and optimize the existing ones. When a new catalyst is synthesized, the first step is to select the chemical elements by means of mathematical algorithms and discard thosewho are not suitable. For example, choosing 50 chemical compounds the possible combination are thousands from 1,255 for binary up to 230,300 quaternary combination[24]. Before commercialization, the synthesised catalyst is tested on laboratory scale and then into a pilot plant under different operating conditions. The reactors (fixed, fluidized bed reactors etc.) used in the experimental tests affect the shape and texture of catalysts (pellets, spherical, granular particles etc.). In the following section, for example, the most recent catalysts developed by BASF and Clariant are described:
- FortressTM NXT is the catalyst worked out by BASF for Fluid Catalytic Crackyng (FCC). It allows to increase metals passivation reducing coke and hydrogen production.[25]
- PolyMax 850 is launched by Clariant. It is a new phosphoric acid that converts olefins to gasoline and solvents. The catalyst allows to reduce CO2 emissions of about 100,000 tons compared to common ones. It can be recycled and use as fertilizer or phosphoric substance.[26]

Figure 5 – (A) FortressTM NXT (25), (B) PolyMax 850 (26)
4.Conclusions
From the first large-scale plants for the production of sulfuric acid in 1875, catalysts have been a fast diffusion in the industrial processes.[27] In the chemical sectors they are used for the production of several compounds such as xylene, ethylbenzene, cumene and so on. In refining processes, they are used in hydrotreating, catalytic reforming, isomerization synthetic fuels, catalytic dewaxing etc. Nowadays about 80-90%24 of chemical processes adopt catalysts (mainly heterogenous catalysts) and the global market for the production/regeneration reach billions of US$. Therefore,is necessary to develop new catalysts and optimize the selectivity and activity of existing ones by reducing the deactivation processes.