Author: Mauro Capocelli, Researcher, University UCBM – Rome (Italy)
1. Theme description
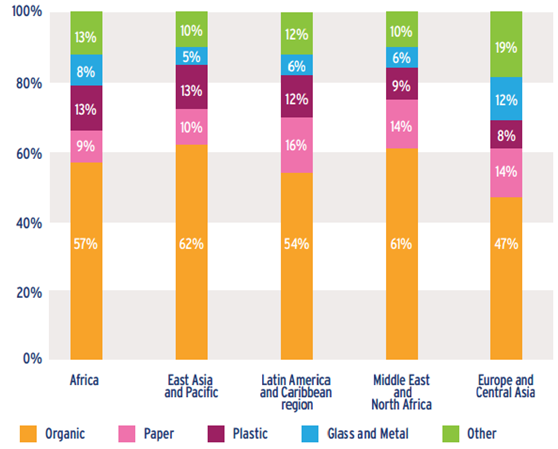
The growth of economy and consumes, combined with the modern models of production, have resulted in a constant increasing generation of waste plastics. The global production of municipal solid wastes (MSW) overcame the value of 3·106 ton/day and should almost double in 2025 [1]. The plastic waste (PSW) represents more than the 10% in the industrialized as well in the less developed countries (see Figure 1). The plastic production has overcame the 200 millions of tonnes in 2007 and is increasing at a rate by 5% per year [2]. The highest percentage is given by containers, packaging, soft drinks, commonly synthetized using non-renewable resources. In this frame, the recycle of plastic represents a challenge as well as a great opportunity, with the aim of save land (used for disposal) and reduce the production impact, i.e. raw-material utilization and emissions of greenhouse gases. At the present, an increasing effort has been devoted to the development of recycling techniques and integral strategies. In 2012, North Americans generated about 251 million tons of trash and recycled (and/or composted) almost the 35% of it [3]. The existent recycling pathways are represented in Figure 1; each of them provides unique advantages that make it suitable for specific applications, locations and requirements. The mechanical recycling (primary through re-extrusion of selected materials and secondary from mixed feedstock) involves physical treatment aimed at reinserting the materials into the production cycle with low energy consumption and almost zero pollutant emission. The thermochemical conversion techniques involve the complete or partial oxidation of the material producing heat, power and/or gaseous fuels, oils and chars. These kind of treatments generates by-products that must be treated and/or disposed. The conversion methods of waste plastics into fuel (tertiary) depend on the types of plastics to be targeted and is commonly realized through processes schemes involving gasification and pyrolysis. In general, the conversion of waste plastic into fuel requires non-hazardous and combustible feedstock. In this field, the incineration technique with energy recovery (quaternary) is the major competitor obtaining the best efficiencies with, on the higher hand, a severe impact on the environment due to release of harmful gases like dioxins, hydrogen chloride, particulate matters and carbon dioxide. In the thermochemical conversion process it is also possible to combine different feedstocks in hybrid schemes exploiting the value of low quality fuels.
Figure 1 – Composition of Municipal Solid Waste in 2012 by regions [4]
Figure 2 – Plastic Solid Waste recycling scheme (adapted from Panda et al., 2010) [5]
The available consolidated methods for the conversion of plastics to liquid fuel are primarily based on the pyrolysis and/or gasification processes producing three different phases: a solid phase (char, 5–25 wt%), a liquid phase (tar, 10–45 wt%) and a gas phase [6]. The primarily produced C20–C50 hydrocarbon are cracked in the gas phase to obtain lighter hydrocarbons, as ethane and propene, which are unstable at high temperatures and react to form aromatic compounds as benzene or toluene. Higher temperatures (above 500°C) and longer residence time disadvantage the tar formation and increase the production of coke, methane and hydrogen. The chloride content in the feedstock and the fluid dynamics are the main parameters to control. The oils produced from plastics have high calorific value, comparable to that of gas oil derived from petroleum (see Table 1, extracted from the related Bulletin of the Royal Society of Chemistry [7]).
Table 1 – Fuel properties of oils derived from the pyrolysis of various wastes
2. Applications
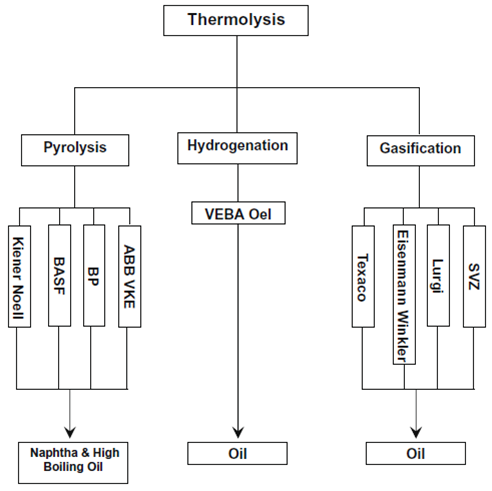
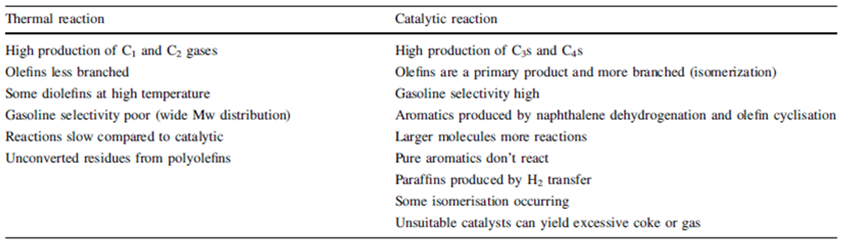
The application of thermo-chemical conversion can be represented through the scheme propose by Mastellone et al., 1999 [8]. Pyrolysis consists of the thermal decomposition under an inert gas like nitrogen; the typical scheme is reported in Figure 3. The plastic materials are introduced into a reactor where they decompose at 400°C-600°C; the major product of the process is an oily mixture of liquid hydrocarbons obtained through the condensation of the decomposed vapours. The evaporated oil may also be further cracked with a catalyst. The boiling point of the produced oil is controlled by the operation conditions of the reactor, the type of reactor, and presence of catalyst. Commonly, the hydrocarbons with high boiling points such as diesel, kerosene and gasoline are then fractionated through fractional distillation. The two main problems associated with thermal cracking are the conversion limits and large molecular weight distribution in the pyrolysis product, resulting in limited market value. In this frame, the use of catalysts enable to decrease the cracking temperature and/or to increase the reaction rate as well as to increase the selectivity and consequently the product quality. The main advantages of the catalytic process are reported in Table 2, while the main drawback is the loss in catalyst activity due to coke formation.
Figure 2 – Scheme of thermolysis process of Plastic Solid Waste [9]
Table 2 – Comparison of thermal and catalytic cracking [10]
One of the first widespread technology for pyrolysis appeared in 1978 with the name of PYROPLEQ. It is based on pyrolysis at circa 500 °C in an externally heated rotary drum and gas combustion at 1200 °C. The system has been coupled with a co-generative rankine cycle (2.2 MW) and alimented with PSW. The BP process consists of a fluidized bed heated at 500 °C in the absence of air; the decomposition generates hydrocarbon vapors with a high content of monomers as and a relative low methane percentage and pollutants as HCl which has to be neutralized by lime adsorption. The yield of liquid fuel production is 85% while the solids produced are typically up to 0.2 kg/kg of total solids feed. The system has been established in Scotland with a capacity of 25,000 tonnes/year after a series of pilot trails. The BASF process started in 1994 with a pilot plant capacity of 15,000 ton/year in Germany. It consists of a pre-treatment stage (grinding and separation) and a multi-stage melting and reduction process. The HCl is processed in the hydrochloric acid production plant. The NKT process realizes the thermal conversion of waste mixtures in a reactor at a low pressure (2–3 bars) and a moderate temperature (375 °C) and has proven to be succsfull for PSW treatment, especiallin for PVC cables [11].
The gasification is achieved by reaction at high temperature (600-800°C) with a controlled amount of oxygen and/or steam (the gasifying agent). The air factor is generally 20% – 40% of the amount of air needed for the combustion of the PSW.[12] The process oxidizes the hydrocarbon feedstock to generate the endothermic depolymerisation heat and produce (primary) a gaseous mixture of carbon monoxide and hydrogen, with minor percentages of gaseous hydrocarbons. The produced syngas can be used as a source of energy for combustion processes or as a source of chemical building blocks from which chemicals may be manufactured. The Texaco process, sketched in Figure 4, represents one of the major industrial example of PSW gasification. It was firstly tested at the pilot scale in the USA (10 ton/day) in 1997. In the liquefaction step, the plastic waste is cracked (depolymerisation in mild conditions) into a synthetic heavy oil and a gas fractions. In the second step oil and condensed gas are injected to the entrained gasifier with oxygen and steam at 1200-1500 ºC. The gasification pressure can be adjusted to the pressure of the served downstream process. The syngas contains small amounts of CH4, CO2 and inert besides the main components CO and H2 and is treated to remove HCl and HF and. In recent years, the gasification of plastics has been intensively conducted with useful results. Many research studies are focusing on fluidized bed, co-gasification [13] and two stage gasification in order to overcome the related technological issues: handling of feedstocks, variability of the feedstock physical characteristics, low heating values and tar formation.
Figure 3 – Apparatus for pyrolysis of waste polymers
(1. Transportation, 2. Selective collection, 3. shredding, 4. Washing, 5. Drying, 6. Waste storage, 7. Catalyst storage, 8. Reactor, 9. Heating gas storage, 10. Separation unit, 11. Catalyst filter) [14].
Figure 4 – Texaco gasification process schematic diagram [15]
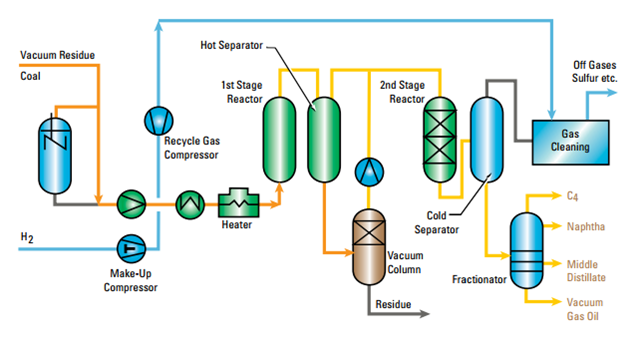
The chemical conversion technique can also count on the hydrogenation process. It represents a potential alternative for breaking down the long polymer chains generating highly saturated products and avoiding the presence of olefins in the liquid fractions. Moreover, compared to the other treatments, the hydrogen promotes the removal of Cl, N and S. Main drawbacks are related to the supplying of hydrogen and the need to use a catalyst and/or operate at higher pressure. The main technology applied in PSW recycling via hydrogenation technology is based on the Veba process, born in the field of the coal liquefaction technologies. The Veba Combi-Cracking [16] (VCC™) is a slurry phase hydrogenation process with integrated hydrocracking for converting petroleum residues at very high conversion rates (>95 wt%, 524 ºC). The process, as described in the Veba report [17], converts the solid hydrocarbons into light distillates by hydrogen addition through two stage reactors detached by a hot separator that recovers the unconverted high boiling material and the additive; the bottom product is fed into a vacuum distillation for recovery of dissolved distillates from the residue. The recovered products are fed to the distillation step together with the top products of the hot separator and are therefore sent to the second hydrotreating/hydrocracking stage in a a catalytic fixed bed reactor (see Figure 5).
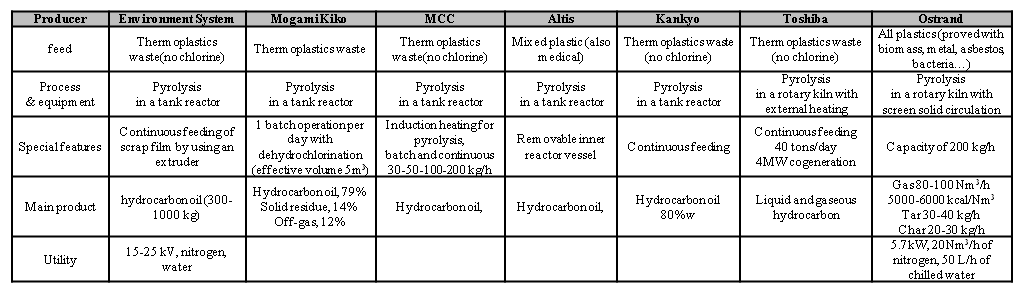
Worth of mention is the project “Converting Waste Plastic into Fuel” by the United Nation Environmental Programme (UNEP) [18]. The UNEP also compiled in 2009 a compendium of the industrial plants appeared in this field, with a detailed list of technology providers. A list of the technologies, together with the related main features has been extracted from the UNEP compendium [19] and reported in the Table 3.
Figure 5 – VCC™ Process Flow Diagram [20]
Table 3 – Fuel production plants
_______________________________