Author: Mauro Capocelli, Researcher, University UCBM – Rome (Italy)
1. Theme description
Global energy demand has dramatically increased in last years and most of the energy needs of the world today (>80%) is still covered by conventional fossil fuels such as coal, petroleum and natural gas (Table 1). The issues of energy efficiency in the fuel production/combustion and storage depletion as well as the increasing concerns about climate changes and environmental pollution related to conventional fuels, are driving the industrial R&D towards the development of alternative solutions. On this basis, this brief review focuses on the most recent strategies in the field of alternative fuel solutions with a specific insight into the low emission strategies of the automotive industry.
Table 1 – World primary energy consumption and percentage of share [1]
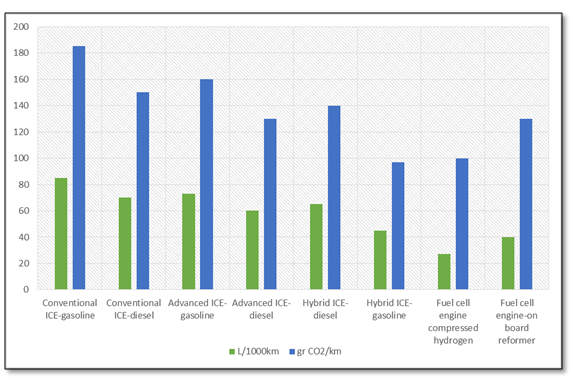
The emissions of the conventional fuel combustion are characterized mainly by the presence of carbon monoxide (CO), nitrogen oxides (NOx), Sulfur oxide (SOx), hydrocarbons and Particulate matter (PM). NOx are harmful to human health and act as precursor of tropospheric ozone. Acute CO poisoning can lead to high toxicity of the central nervous system and heart while a chronic exposure causes depression, confusion and memory loss. Carbon monoxide poisoning mainly causes hypoxia by combining with hemoglobin to form carboxyhemoglobin in the blood reducing the oxygen-carrying capacity of the blood. An exposition to more than 20 ppm SO2 can cause death; moreover SOx pollution strongly affects the life of entire ecosystems for the climate influence. Any recent medical research suggests that PM is among the most dangerous pollutants; the effects of its inhalation (both acute and chronic) is nowadays associated with the majority of respiratory diseases, from asthma to lung cancer and also to cardiopulmonary mortality, premature delivery, birth defects, and premature death.[2] Besides these strong environmental impacts, any conventional fuel contributes to generate greenhouse gas emissions causing the well-known climate changes. Wondering what happens when oils will runs out, Prof. Chris Rhodes asserts that, although the world supply crude oil isn’t going to run out any time soon, it is impossible to follow the current production rate: “ from 1965 to 2005, we see that by the end of it, humanity was using two and a half times as much oil, twice as much coal and three times as much natural gas, as at the start, and overall, around three times as much energy: this for a population that had “only” doubled. Hence our individual average carbon footprint had increased substantially – not, of course, that this increase in the use of energy, and all else, was by any means equally distributed across the globe”[3]. Following the Kyoto Protocol and the subsequent national directives, the industrialized countries are setting stringer regulation policies of emission control for stationary and mobile sources. The main strategies for the development of low-emission vehicles (LEV) are the realization of alternative low-emission fuels for the conventional internal combustion engine vehicle (ICEV) and the development of new high-tech renewable LEV as hybrid and fuel cell vehicles (Fig. 1)[4]. Although these latter are making promising step-forward towards the commercialization[5], they have not still led to a considerable market because of economic, politic and technological barriers. This results in the persistence of ICEV as dominant design. Therefore, this design is the focus of recent R&D effort in order to decrease polluting emissions and to increase energy efficiency of engines (by developing injection, combustion chambers and ignition controlling technologies) as well as by ideating alternative (and more environmental friendly) fuels.
Figure 1 – Flame and flameless firing of heavy fuel oil
(left: flame mode – right: flameless) after Oltra and Saint Jean, 20093
2. Emulsions
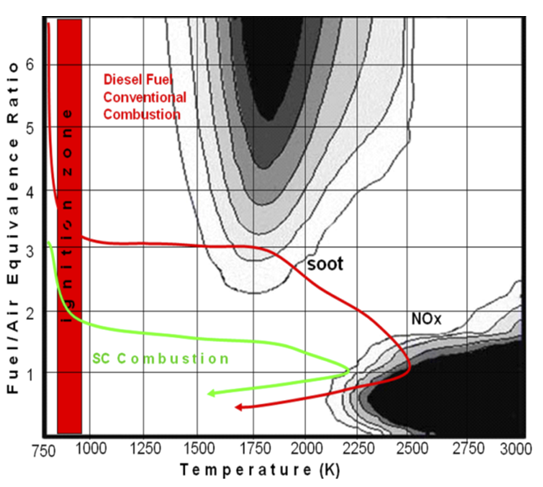
The main pollutants in diesel emission, NOx and PM, have peculiar mechanisms of formation hindering the simultaneous reduction of both and making necessary a trade-off between the two possible pollutant emissions. Lowering the combustion flame temperature in order to reduce NOx generally causes disequilibrium in the balance of soot formation and burnout resulting in an increase in PM emissions. On the other hand, particulate emissions can be reduced by increasing the combustion temperature, an operation that results in increased NOx emissions.
Figure 2 – Regimes of soot and NOx formation expressed in terms of flame equivalence ratio (fuel:air ratio) and flame temperature.
One way to overcome this issue is to replace the fuel with emulsions of diesel oil and water (without retrofitting the engine system). Lif and Holmberg gave an extended review of the water in diesel related system[6]. Water emulsions in oil (W/O) are prepared by using surfactants through mechanical (and ultrasonic[7]), chemical[8], or electric homogenizing machine (i.g. water stirring into microdroplets in oil layers). Surfactants, thanks to the presence of both lipophilic and hydrophilic groups, can reduce the oil and water surface tension creating oil-in-water or water-in-oil two phase emulsions (layer of ionic surfactants can also prevent droplet merging). When the emulsion is heated, water droplets vaporizes breaking out the oil layer (microexplosion). The secondary atomization increases the superficial area of the fuels and air and mixing extent. Secondly, the presence of water dilutes the nuclei of soot growth limiting the soot growth rates. Moreover, the presence of water could enhance soot burnout by increasing the presence of oxidizing species. All these cited aspects can contribute to lower the PM emission inhibiting both soot and ash formation. On the other hand, the high latent heat of vaporization of water will act to lower the temperature causing the NOx emission reduction[9]. Nadeem et al. compared the engine and emission performances of emulsified fuels (5–15% of water) using conventional (CS) and gemini surfactants (GS). Their experimental results highlights the potentiality of W/O to significantly reduce the formation of thermal NOx (from more than 700 to 500 ppm), CO, SOx, soot, hydrocarbons and PM (more than 70% of reduction) in the Diesel engines[10].
3. Fuel desulphurization
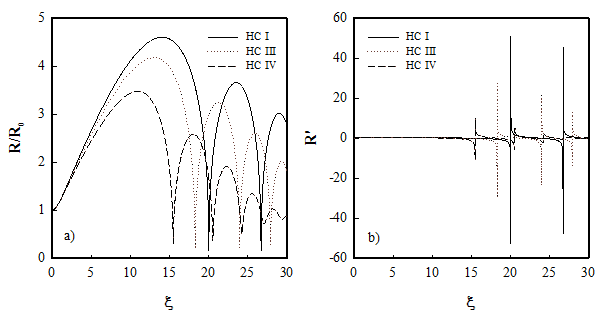
Conventional techniques for desulfurization of transportation fuels are based on hydro–desulfurization (HDS), in which the sulfur in the fuel is removed as H2S. This technique has the drawbacks of limited efficiency for the low reactivity of benzothiophene and dibenzothiophene and high costs for the operating conditions and the hydrogen implementation. On the other hand, the oxidative desulfurization is based on the conversion of non-polar aromatic hydrocarbons containing sulfur to corresponding sulfones, easily extractable with methanol. This liquid–liquid heterogeneous system, which depends on the mass transfer between the interface, can be enhanced through cavitation, both Ultrasonic and Hydrodynamic. Cavitation is the nucleation, growth, and transient collapse of micrometric gas-vapor bubbles driven by a pressure variation. It induces physical and chemical effects[11] in the reaction system that enhance the kinetics and yield of the process (both mechanical and chemical). The chemical effects are in terms of the generation of radicals through the dissociation of gas and vapor molecules during the transient collapse of the cavitation bubbles. The physical effects in terms of turbulence generation and therefore viscous dissipative eddies, shock waves and microjets can be exploited to create emulsion by reducing the mass transfer limitations[12]. A brief description of ultrasonic desulfurization is given by the Sulphco, Inc., a Nevada corporation; it reported great results in the enhancement of fuel desulfurization showing an impressive translation to sulfone concentration through an innovative treatment with ultrasonic horns[13]. Several innovative application of cavitation desulfurization, from patents to applied research and technology development, appeared in the literature[14]. While in the acoustic cavitation, the pressure variation is given by ultrasonic waves, in the hydrodynamic one, it is realized through properly designed flow restriction operating at different pressures and flow rates. An example of the bubble dimensions and shear stresses at the collapse stage is shown in Figure 3.
Figure 3 – Simulation of bubble radius and bubble wall velocity for different configuration of hydrodynamic cavitation operating parameters[15].
4. Alternative fuels
The generally shared belief that the upcoming shortage of oil will accelerate the switch to alternative fuels, all the major oil and automotive companies have alternative fuels research programs[16]. Moreover, the R&D in alternative fuels is often related to environmental friendly strategies. The term alternative fuels comprises hydrogen, compressed natural gas (CNG) and liquefied petroleum gas (LPG), biogas, dimethylether (DME), alcohols such as methanol and ethanol, liquefied petroleum gas (LPG), vegetable oils and fatty acid methyl esters, and blends of these with gasoline or diesel. Therefore, there are different opinions and an ultimate decision about which type of products will dominate the market for vehicle fuels in the future is uncertain and depends on political as well as economic considerations. As visible from the Figure 4, indeed the cost of alternative fuels (ethanol produced from corn in the U.S.) often follows the cost of the equivalent conventional one which is gasoline, the principal market competitor (and rarely is strictly connected to the prices of raw matherials). Generally, the fuels generation can follow the pathway of Natural gas, Biomasses or Electricity. Natural gas is a versatile fuel, employable in modified spark-ignition engines or in dedicated engines[17]. It can be used directly in compressed or liquefied form and converted to methanol, dimethyl ether (DME), gas-to-liquid (GTL) fuel or Fischer–Tropsch diesel. Both PM and NOx emissions from natural gas-derived fuels are very low while sulphur emissions is usually negligible. Liquefied petroleum gas (LPG) is mainly composed by propane and butane (and homologues liquefying at ~800 kPa) and released during the extraction of crude oil and gases of oil refining processes. LPG fuels are based on light low-carbon, clean-burning hydrocarbons and their implementation can bring to substantial reductions of CO, NOx, hydrocarbons and emissions of greenhouse gases. DME (born as an ignition improver of methanol) can be produced from different feedstock such as natural gas, coal, oil residues and biomasses. It has good ignition properties (high cetane number and low auto-ignition temperature); moreover its simple chemical structure and high oxygen content result in soot-free combustion in engines[18].
Figure 4 – Biodiesel Production (millions of gallons/year) in the top world countries (2013) extracted from the 2013 Renewable Energy Data Book[19]
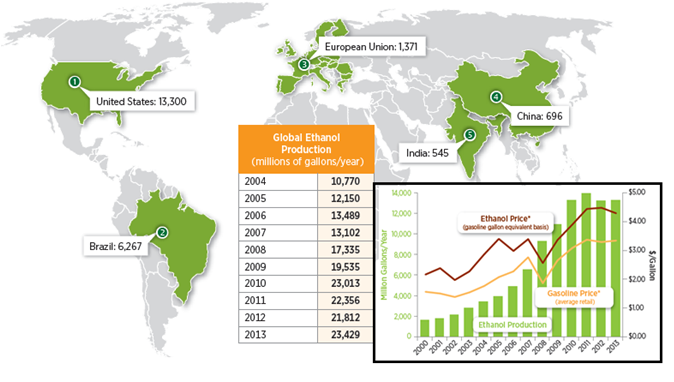
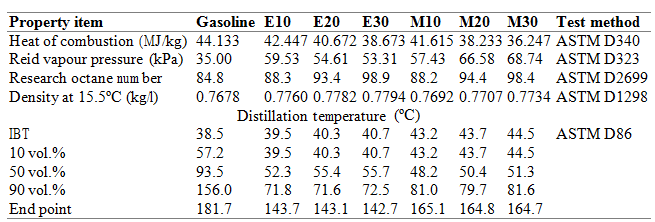
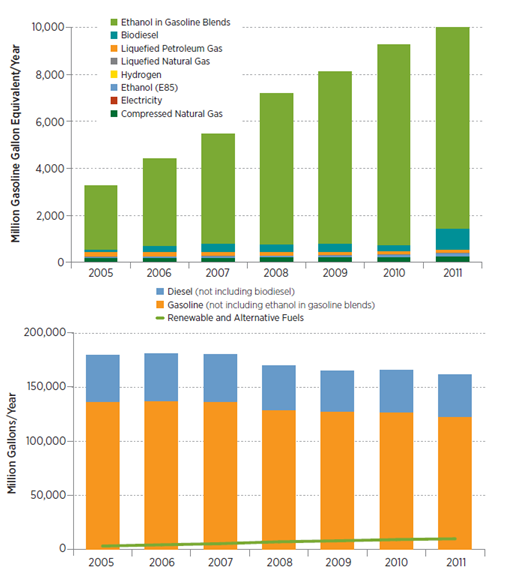
Arcoumanis et al. gave a review of the potential benefits of using DME as alternative fuel in standard compression-ignition engines with slight modification of the conventional system (paying attention to the corrosion and low lubricity related issues)[20]. Hydrogen can be used as a fuel in internal combustion engines and in fuel cells with zero pollutant emission. It can be produced from natural gas as well as water electrolysis. From the economic point of view, its utilization is controlled by the cost and the source of electrical energy. Toyota Mirai, the first commercialized fuel cell car, is recently finding a great success highlighting that the spreading of such kind of technologies is limited only by infrastructural issues: distribution chain, storage and handling (both in vehicles and at gas stations). Although these issues are not yet overcome, hydrogen represents a concrete frontier for the automotive industry. The biomass for fuel production can have various origins, such as black liqueur, forestry residues, or municipal or industrial waste products. The resulting fuels are. Among all the different biomass based fuels, the most accessible ones today are diesel and ethanol. Other resulting fuels are methanol, DME and Fischer-Tropsch diesels while the gasification processes of biomass results in biogas-to-liquid fuels. Biodiesel is conventionally made by transesterification of a triglyceride with methanol (fatty acid methyl ester). It can be used either pure or as blends with regular diesel with the benefit of reduced CO, CO2 hydrocarbon and PM emissions. Biodiesel combustion produces higher NOx emission (to be treated with improved catalytic filters) while reduces the SOx emission to almost zero. Rape seed and sunflower are among the main source of biodiesel edible raw material. To minimize the reliance on edible vegetable oil and to exploit the naturally available oil plants, Ashraful et al. studied the fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils (karanja, mohua, rubber seed, and tobacco biodiesel) providing a detailed extensive review in this field[21]. Based on their findings (reduce CO, HC and smoke emission) they asserts that non-edible oils have the potential to replace edible oil-based biodiesels in the near future (some controversy arise from the NOx point of view). In 2013 the total biodiesel production was 6,948 millions of gallons with an increase of the 17% from 2012 to 2013. In 2013 the United States led the world in biodiesel production, followed by Germany, Brazil, Argentina, and France and Indonesia (U.S. cost of 3.92 $/gall in 2013)[22]. Because of the reduction of PM emission in oxygenated fuels, the alcohols are particularly attractive as alternative to the conventional ones. Gravalos et al. described the Performance and Emission Characteristics of Spark Ignition Engine Fuelled with Ethanol and Methanol Gasoline Blended Fuels highlighting the mixture properties (reported in Table 2)[23]. Moreover, they can be produced as biofuels (also not linked to the food production). In 2013, the Indian River BioEnergy Center began producing cellulosic ethanol at commercial volumes for the first time and now is among the major technology center in the field of bioenergy. Its goal is to << take wastes and sustainably turn them into advanced biofuel and renewable power>>[24]. Methanol can be produced from coal, biomass or even natural gas while ethanol mainly from sugar cane, starch wheat or wine. All car manufactures have approved the use of E10, a blend of 10% ethanol and 90% gasoline and E5, blend of 5% ethanol and 95% gasoline in the ordinary gasoline cars and these blends are commonly available in the US and in Europe. In Brazil the majority of the cars utilizes neat ethanol or lower level blends produced from sugar cane while in the U.S. the ethanol production (13,300 million gallons in 2013,) is mainly based on corn. In 2013 the U.S. led the world market (57% of the overall production) followed by Brazil at 27% and E.U. at 6% (see Figure 3). To understand the order of magnitude of the number reported above, Figure 5 shows the data (taken from the U.S. Department of Energy report[25]) related to the consumption of renewable and alternative fuel (top) with a comparison to the consumption of traditional fuel (bottom) in the United States (for the year 2013).
Table 2 – Properties of different ethanol and methanol gasoline blended fuels (extracted from ref. 20)
Figure 5 – Consumption of renewable and alternative fuel (top) and of traditional fuel (bottom) in the United States (for the year 2013).
________